The real estate landscape is experiencing a dramatic transformation in 2026, with specialized properties moving from the periphery to center stage. Life sciences facilities, biotech laboratories, and cold storage warehouses are no longer niche investments—they've become essential infrastructure driving economic growth and innovation. For property professionals, understanding how to properly survey and evaluate these highly technical spaces represents both a challenge and an opportunity. Surveying Niche to Essential Real Estate: Strategies for Labs, Cold Storage, and Life Sciences Sites in 2026 requires specialized knowledge that goes far beyond traditional residential or commercial property assessment.
The life sciences real estate sector has undergone significant market correction following unprecedented expansion. The construction pipeline has contracted from a peak of 37 million square feet in 2023 to just 6 million square feet in 2026—the lowest level since early 2019.[1] This dramatic shift signals that the speculative building phase has ended, with future construction limited primarily to fully leased build-to-suit projects.[1] For surveyors and property professionals, this evolving landscape demands new approaches to property assessment, valuation, and strategic planning.
Key Takeaways
- 🔬 Specialized survey techniques are essential for life sciences properties, requiring understanding of cleanroom standards, temperature controls, and biosafety requirements
- 📊 Market stabilization is underway with vacancy rates expected to plateau at 23.3% in 2026, creating opportunities for strategic acquisitions and repositioning
- 🏭 Manufacturing-focused facilities maintain above 90% occupancy nationwide, significantly outperforming pure R&D spaces and representing lower-risk investments[3]
- 💡 AI-native biotechs use 25% less space per employee and 15-20% less wet lab space, fundamentally changing facility design and survey considerations[2]
- 📈 Capitalization rates have expanded from 4.4% to 6.6% since early 2022, reshaping valuation approaches and investment strategies[3]
Understanding the Life Sciences Real Estate Transformation

From Niche to Essential Infrastructure
The life sciences sector has evolved from a specialized subset of commercial real estate into a critical component of national infrastructure. This transformation reflects several converging trends: increased pharmaceutical onshoring, expanding contract manufacturing operations, and the integration of artificial intelligence into biotech research. More than a dozen of the largest pharmaceutical companies have announced major U.S. manufacturing investments in recent years, driven by supply chain resilience concerns and policy incentives.[3]
For property surveyors, this shift means that specialized life sciences facilities now require the same level of professional scrutiny and expertise traditionally reserved for major commercial developments. The technical complexity of these properties demands surveyors who understand not just structural integrity and building systems, but also regulatory compliance, contamination risks, and specialized equipment integration.
Market Dynamics Reshaping the Sector
The current market presents a fascinating paradox. While vacancy rates have climbed to cyclical peaks—with major hubs like Boston (80.1%), San Francisco (79.0%), and San Diego (73.3%) experiencing substantial declines from mid-90% occupancy levels in 2022[3]—the long-term fundamentals remain compelling. The State Street SPDR S&P Biotech ETF (XBI) surged dramatically in the second half of 2025, historically a leading indicator for increased leasing demand in lab and R&D space.[1]
This market correction has created opportunities for sophisticated investors and developers who understand how to properly assess these properties. Philadelphia has emerged as a standout performer, growing inventory by more than 21% over three years while maintaining relatively stable occupancy levels, significantly outperforming major coastal hubs.[3] This success demonstrates that location strategy and property quality matter enormously in this sector.
Understanding these market dynamics is crucial when conducting stock condition surveys or comprehensive property assessments for life sciences facilities, as the valuation context has shifted dramatically from the speculative peak of 2022-2023.
Surveying Niche to Essential Real Estate: Core Assessment Strategies
Specialized Survey Requirements for Laboratory Spaces
Laboratory facilities present unique challenges that distinguish them from conventional commercial properties. A comprehensive survey of a life sciences facility must evaluate multiple specialized systems and compliance requirements:
Environmental Control Systems 🌡️
- HVAC systems with precise temperature and humidity controls
- Air change rates and filtration standards (HEPA systems)
- Pressure differential maintenance between zones
- Backup power and redundancy systems
- Energy efficiency and operational costs
Cleanroom Classifications
- ISO cleanroom standards compliance (ISO 14644)
- Particle count verification and monitoring
- Surface contamination assessment
- Gowning area adequacy
- Material transfer protocols
Safety and Compliance Infrastructure
- Biosafety level (BSL) certifications and containment
- Chemical storage and handling facilities
- Emergency shower and eyewash stations
- Fume hood functionality and certification
- Waste management systems (chemical, biological, radioactive)
When conducting building surveys on these properties, surveyors must verify that all specialized systems are functioning correctly and meet current regulatory standards. Unlike residential properties where a RICS Home Survey focuses primarily on structural condition, life sciences facilities require deep technical expertise across multiple engineering disciplines.
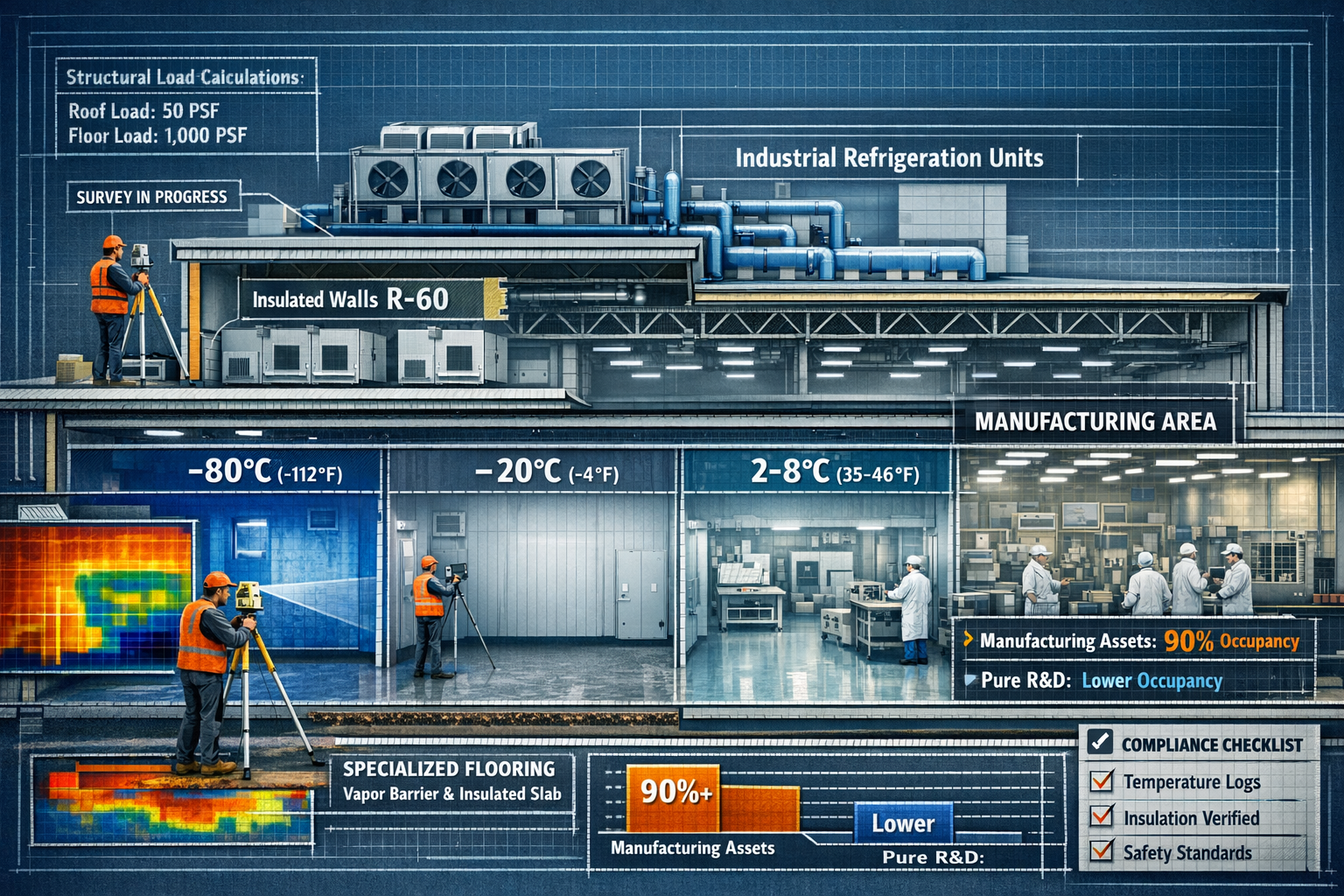
Cold Storage and Temperature-Controlled Facilities
Cold storage facilities represent another critical component of the life sciences ecosystem, particularly for pharmaceutical manufacturing and distribution. These properties require specialized survey approaches that address:
Thermal Envelope Integrity
- Insulation R-values and thermal bridging
- Vapor barrier continuity and condition
- Door seals and loading dock thermal protection
- Roof membrane condition and drainage
- Floor insulation and heating systems
Refrigeration Systems Assessment
- Primary and backup refrigeration capacity
- Temperature zone capabilities (-80°C to +25°C range)
- Monitoring and alarm systems
- Energy consumption and efficiency ratings
- Maintenance history and remaining useful life
Structural Considerations
- Floor loading capacity for racking systems
- Structural impact of refrigeration equipment
- Foundation performance in freeze-thaw cycles
- Condensation management and moisture control
- Fire suppression system compatibility with cold environments
The market for these facilities has proven remarkably resilient. Life sciences properties with a manufacturing component maintain above 90% occupancy nationwide, significantly outperforming pure R&D facilities.[3] This occupancy differential makes cold storage and manufacturing facilities particularly attractive for investors seeking stable returns, but also demands rigorous survey protocols to justify premium valuations.
Wet Lab vs. Dry Lab Distinctions
Understanding the difference between wet lab and dry lab spaces is fundamental to proper property assessment:
Wet Lab Characteristics:
- Extensive plumbing and drainage infrastructure
- Chemical-resistant surfaces and materials
- Specialized ventilation with high air change rates
- Laboratory-grade electrical systems
- Deionized water and specialty gas distribution
- Higher construction and fit-out costs ($600-$1,200+ per square foot)
Dry Lab Characteristics:
- Computational and analytical focus
- Standard office-grade infrastructure with enhanced power
- Lower air change requirements
- Easier conversion to alternative uses
- Lower construction costs ($300-$600 per square foot)
- Greater flexibility for AI-native biotech companies
The emergence of AI-native biotechnology companies has fundamentally altered space utilization patterns. These organizations use approximately 25% less space per employee and 15-20% less wet lab space compared to traditional biotechs.[2] For surveyors, this trend suggests that adaptability and convertibility should be weighted more heavily in property assessments, as future tenants may have very different space requirements than current occupants.
When comparing different types of survey approaches for these facilities, the complexity and technical requirements clearly necessitate specialized expertise beyond standard commercial property assessment.
Advanced Valuation Considerations for Life Sciences Properties
Capitalization Rate Evolution and Investment Metrics
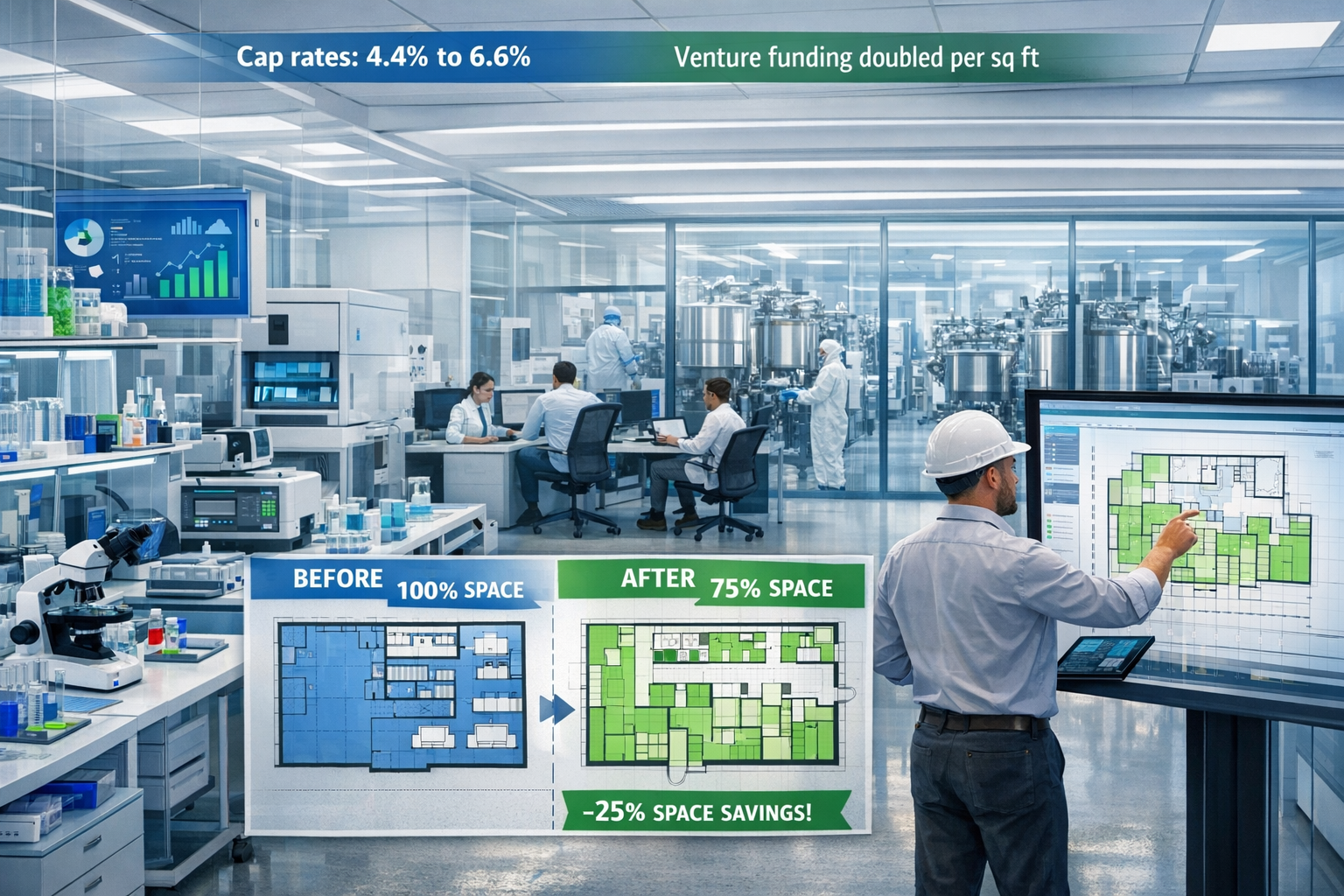
The investment landscape for life sciences real estate has transformed dramatically. Capitalization rates have climbed from 4.4% in early 2022 to 6.6% in the most recent quarter (as of mid-2025), reflecting both rising interest rates and increased risk perception.[3] This 220-basis-point expansion has profound implications for property valuation and survey priorities.
Key Valuation Drivers:
| Factor | Impact on Value | Survey Focus |
|---|---|---|
| Tenant Credit Quality | High | Lease documentation review, tenant financial health |
| Specialized Systems Condition | High | Detailed M&E assessment, remaining useful life analysis |
| Regulatory Compliance | Critical | Certification verification, compliance documentation |
| Conversion Flexibility | Moderate-High | Structural adaptability, infrastructure modularity |
| Location/Cluster Proximity | High | Market analysis, accessibility to talent and services |
The capital efficiency trend is particularly noteworthy. Venture funding per leased square foot in the U.S. has doubled over eight years as investors demand greater efficiency from biotech companies.[2] This means that newer facilities designed for capital-efficient operations may command premium valuations despite smaller footprints.
When conducting RICS valuations for life sciences properties, professionals must incorporate these sector-specific metrics alongside traditional comparable analysis. The standard approaches used for conventional commercial properties may significantly misvalue these specialized assets.
Build-to-Suit vs. Speculative Development Assessment
The shift away from speculative construction toward build-to-suit projects represents a fundamental market evolution. Remaining speculative lab/R&D space will be delivered in 2026, with any future construction limited to fully leased build-to-suit projects.[1] This transition affects survey priorities:
Build-to-Suit Advantages:
- ✅ Tenant-specific customization and efficiency
- ✅ Long-term lease security (typically 10-15 years)
- ✅ Higher initial occupancy certainty
- ✅ Purpose-built systems matching exact requirements
- ✅ Lower vacancy risk during economic downturns
Build-to-Suit Survey Considerations:
- Verify alignment between installed systems and lease specifications
- Assess over-customization risk for future tenants
- Evaluate tenant improvement allowance utilization
- Review performance guarantees and warranties
- Analyze lease structure and renewal options
Speculative Development Challenges:
- ⚠️ Generic specifications may not match tenant needs
- ⚠️ Extended lease-up periods in current market
- ⚠️ Higher vacancy risk and carrying costs
- ⚠️ Potential need for significant tenant improvements
- ⚠️ Market timing risk with 18-24 month construction cycles
For surveyors assessing existing speculative developments, particular attention should be paid to adaptability features that allow cost-effective customization for various tenant types. Properties with modular infrastructure, flexible floor plates, and convertible lab/office ratios will likely outperform rigid, single-purpose facilities.
Geographic Market Variations
Location analysis has become increasingly sophisticated in the life sciences sector. While traditional hubs like Boston, San Francisco, and San Diego continue to dominate, emerging markets are demonstrating compelling performance characteristics:
Tier 1 Markets (Established Hubs):
- Boston/Cambridge: Deepest talent pool, highest rents, significant new supply absorption challenge
- San Francisco/South Bay: Innovation ecosystem, high costs, occupancy pressure from overbuilding
- San Diego: Strong research institutions, biotech concentration, similar oversupply concerns
Tier 2 Markets (Emerging Strength):
- Philadelphia: Strong performance despite inventory growth, competitive costs, research university access
- Research Triangle (NC): Growing pharmaceutical presence, favorable business climate, talent pipeline
- Seattle: Tech-bio convergence, AI integration, strong venture capital access
When conducting commercial building surveys in different markets, regional variations in construction standards, utility costs, labor availability, and regulatory environments must be factored into the assessment. A facility that represents excellent value in Philadelphia might be considered substandard in Boston/Cambridge due to different market expectations and tenant requirements.
Surveying Niche to Essential Real Estate: Technical Due Diligence Protocols
Comprehensive Building Systems Assessment
Life sciences facilities demand extraordinary attention to mechanical, electrical, and plumbing (MEP) systems. These systems often represent 40-60% of total construction costs and are critical to property functionality and value retention.
Mechanical Systems Deep Dive:
🔧 HVAC Performance Verification
- Design air change rates vs. actual performance (typically 6-20 ACH for labs)
- Temperature and humidity control precision (±1°C, ±5% RH common)
- System redundancy and fail-safe mechanisms
- Filter efficiency and replacement schedules
- Energy recovery systems and efficiency metrics
Electrical Infrastructure Analysis:
- Power density requirements (often 15-25 watts/sq ft for labs vs. 5-7 for offices)
- Backup generator capacity and testing protocols
- Uninterruptible power supply (UPS) systems for critical equipment
- Electrical panel capacity and expansion potential
- Emergency power distribution and switching systems
Plumbing and Process Systems:
- Deionized (DI) water generation and distribution
- Reverse osmosis (RO) water systems
- Specialty gas distribution (nitrogen, CO2, compressed air)
- Acid waste neutralization systems
- Vacuum systems and compressed air quality
A thorough structural survey must go beyond basic structural integrity to evaluate whether the building can support the extraordinary MEP loads and future modifications typical in life sciences facilities. Floor-to-floor heights, structural loading capacity, and vertical distribution pathways for utilities are critical factors that significantly impact property value and tenant suitability.
Environmental and Contamination Assessment
Life sciences facilities present unique environmental risks that require specialized investigation:
Phase I Environmental Site Assessment Enhancements:
- Historical use of hazardous materials and chemicals
- Biological agent handling and containment history
- Radioactive material storage and use
- Waste stream documentation and disposal records
- Spill history and remediation documentation
Specialized Testing Requirements:
- Surface contamination sampling in former lab areas
- Air quality testing for residual chemical vapors
- Soil and groundwater assessment near waste storage areas
- Asbestos and lead assessment (common in older research facilities)
- Radon testing (particularly important for underground facilities)
The Contract Research and Manufacturing Organization (CDMO) sector has seen revenues at large publicly traded companies roughly double over five years,[2] indicating increased outsourcing of manufacturing operations. Many CDMO facilities involve chemical synthesis and manufacturing processes that create elevated environmental risk profiles. Properties with CDMO history require particularly rigorous environmental due diligence.
For properties with identified contamination issues, a specific defect report focused on remediation requirements and costs becomes essential for accurate valuation and transaction structuring.
Regulatory Compliance and Certification Verification
Life sciences facilities operate under extensive regulatory oversight that directly impacts property value and usability:
Key Regulatory Frameworks:
📋 FDA Compliance (for pharmaceutical manufacturing)
- Current Good Manufacturing Practice (cGMP) standards
- Facility design and construction documentation
- Validation protocols and documentation
- Change control procedures
- Audit history and corrective actions
Biosafety and Containment:
- CDC/NIH Biosafety Level certifications (BSL-1 through BSL-4)
- Institutional Biosafety Committee approvals
- Select agent registration (if applicable)
- Containment system testing and certification
- Emergency response protocols
Environmental Permits:
- Air emissions permits for laboratory hoods and processes
- Wastewater discharge permits and pretreatment systems
- Hazardous waste generator status and management
- Stormwater management compliance
- Chemical storage permits and reporting (SARA Title III)
Surveyors should verify that all necessary permits and certifications are current and transferable. Lapsed certifications or permit violations can create significant transaction delays and devalue properties substantially. The cost to bring a non-compliant facility up to current standards can easily exceed millions of dollars for larger facilities.
Strategic Considerations for 2026 and Beyond
AI Integration and Space Efficiency Trends
The integration of artificial intelligence into biotechnology research is fundamentally reshaping facility requirements. AI-first startups demonstrate dramatically different space utilization patterns compared to traditional biotech companies, using approximately 25% less space per employee and 15-20% less wet lab space.[2]
Implications for Property Assessment:
Computational Infrastructure Priority:
- High-density computing facilities and server rooms
- Enhanced cooling systems for computational equipment
- Robust data connectivity and bandwidth
- Cloud integration and hybrid computing support
- Cybersecurity and data protection infrastructure
Reduced Wet Lab Footprint:
- More efficient lab layouts with higher utilization rates
- Automation and robotics reducing manual bench space needs
- Centralized core facilities vs. distributed lab space
- Flexible lab modules that can be reconfigured quickly
- Higher office-to-lab ratios than traditional biotechs
Collaboration Space Enhancement:
- Open collaborative areas supporting interdisciplinary teams
- Video conferencing and remote collaboration infrastructure
- Flexible furniture and reconfigurable spaces
- Integration of computational and wet lab areas
- Amenity spaces supporting extended work hours
For surveyors evaluating properties for potential repositioning or redevelopment, understanding these evolving space requirements is crucial. Facilities designed around 2010-2015 paradigms may require significant modification to meet current tenant expectations, while newer facilities built with flexibility in mind may command premium valuations.
Manufacturing and Onshoring Opportunities
The pharmaceutical onshoring trend represents one of the most significant opportunities in life sciences real estate. More than a dozen of the largest pharmaceutical companies have announced major U.S. manufacturing investments, driven by supply chain resilience concerns and policy incentives.[3]
Manufacturing Facility Characteristics:
🏭 Scale and Infrastructure Requirements:
- Larger floor plates (often 50,000-200,000+ sq ft)
- Heavy floor loading capacity (500-1,000+ lbs/sq ft)
- Extensive utility capacity (water, power, steam, compressed gases)
- Waste treatment and environmental control systems
- Logistics and material handling infrastructure
Occupancy Stability:
- Manufacturing-oriented assets maintain above 90% occupancy nationwide[3]
- Longer lease terms (typically 15-20 years for build-to-suit)
- Higher tenant improvement investments creating exit barriers
- Strategic importance to tenant operations reducing relocation risk
- More stable cash flows compared to pure R&D facilities
Survey Priorities for Manufacturing Facilities:
- Process equipment integration and utility connections
- Cleanroom and controlled environment certifications
- Material flow and logistics efficiency
- Expansion capacity and future build-out potential
- Regulatory compliance documentation and history
The resilience of manufacturing-focused facilities during the current market correction demonstrates their investment appeal. When conducting valuation assessments, manufacturing components should be weighted heavily as value stabilizers, particularly in uncertain economic environments.
Adaptive Reuse and Conversion Strategies
With vacancy rates stabilizing at elevated levels (23.3% expected in 2026),[1] adaptive reuse and conversion opportunities are emerging across the life sciences sector:
Conversion Pathways:
Lab-to-Office Conversions:
- Remove specialized lab infrastructure
- Reduce HVAC capacity and complexity
- Standard office finishes and systems
- Potentially lower operating costs
- Broader tenant pool but lower rents
Office-to-Lab Conversions:
- Structural capacity for heavier MEP systems
- Floor-to-floor height adequacy (typically 14'+ required)
- Utility service capacity (electrical, water, gas)
- Zoning and permitting considerations
- Significant capital investment required ($200-$400/sq ft)
Wet Lab-to-Dry Lab Conversions:
- Remove chemical infrastructure while retaining HVAC
- Reduce plumbing and specialty gas systems
- Maintain higher power density than standard office
- Moderate conversion costs ($50-$150/sq ft)
- Retain life sciences tenant pool
When evaluating properties for conversion potential, surveyors should assess both the technical feasibility and economic viability. A detailed building survey should identify structural limitations, required permits, estimated conversion costs, and post-conversion market positioning.
Risk Mitigation and Due Diligence Best Practices
Given the technical complexity and market volatility in the life sciences sector, comprehensive due diligence is essential for risk mitigation:
Enhanced Due Diligence Checklist:
✅ Technical Systems Verification
- Third-party commissioning reports for major systems
- Maintenance records and service contracts
- Equipment age and replacement reserves
- Energy consumption benchmarking
- System redundancy and business continuity capabilities
Financial and Operational Analysis
- Tenant financial strength and funding runway
- Lease structure and renewal probability
- Operating expense reconciliation and CAM charges
- Capital expenditure history and projections
- Property tax assessment and appeal potential
Market and Competitive Positioning
- Cluster dynamics and competitive supply
- Tenant demand drivers and sustainability
- Comparable property analysis and positioning
- Submarket absorption trends and forecasts
- Regulatory environment and policy support
Legal and Compliance Review
- Title and survey verification
- Environmental compliance documentation
- Building permits and certificates of occupancy
- Lease abstracts and tenant estoppels
- Litigation history and outstanding violations
The expanded capitalization rates (from 4.4% to 6.6%)[3] mean that even small differences in risk assessment can significantly impact property values. Thorough due diligence that identifies and quantifies risks allows for appropriate pricing and transaction structuring.
Future-Proofing Life Sciences Real Estate Investments
Sustainability and ESG Considerations
Environmental, social, and governance (ESG) factors are increasingly important in life sciences real estate, driven by both tenant demand and investor requirements:
Energy Efficiency Priorities:
- Laboratory buildings typically consume 5-10x more energy per square foot than office buildings
- High-performance HVAC systems with heat recovery
- LED lighting and occupancy-based controls
- Building automation and energy management systems
- Renewable energy integration (solar, geothermal)
Water Conservation:
- Cooling tower water treatment and recycling
- Low-flow fixtures and water-efficient landscaping
- Process water recycling systems
- Rainwater harvesting for non-potable uses
- Water consumption monitoring and benchmarking
Sustainable Materials and Operations:
- Low-VOC materials and finishes
- Sustainable cleaning and maintenance practices
- Waste reduction and recycling programs
- Green building certifications (LEED, BREEAM)
- Indoor environmental quality optimization
Surveyors should evaluate sustainability features not just as compliance items but as value drivers. Properties with strong ESG credentials increasingly command premium rents and attract higher-quality tenants, particularly among well-funded biotech companies with sophisticated sustainability commitments.
Technology Integration and Smart Building Features
The convergence of life sciences and technology creates opportunities for enhanced building performance through smart systems:
Smart Building Technologies:
💻 IoT Sensors and Monitoring:
- Real-time environmental monitoring (temperature, humidity, pressure)
- Equipment performance tracking and predictive maintenance
- Energy consumption analytics and optimization
- Occupancy sensing and space utilization analysis
- Indoor air quality monitoring and response
Building Management Systems:
- Integrated control of HVAC, lighting, and security
- Remote monitoring and troubleshooting capabilities
- Automated fault detection and diagnostics
- Energy optimization algorithms
- Tenant portal access to building data
Security and Access Control:
- Biometric access systems for controlled areas
- Video surveillance and analytics
- Visitor management and tracking
- Integration with tenant security systems
- Cybersecurity for building systems
When assessing properties, surveyors should evaluate the sophistication and integration of building technology systems. Well-implemented smart building features can reduce operating costs by 15-30% while enhancing tenant satisfaction and retention.
Market Outlook and Investment Timing
The 2026 life sciences real estate market presents a complex but potentially rewarding landscape for informed investors and developers:
Near-Term Outlook (2026-2027):
- Vacancy stabilization at cyclical peak of 23.3%[1]
- Minimal new speculative supply entering market
- Biotech equity market momentum supporting demand recovery
- Cap rate stabilization as interest rate environment clarifies
- Opportunities for value-add acquisitions and repositioning
Medium-Term Trends (2028-2030):
- Gradual occupancy recovery as demand catches up with supply
- Continued shift toward manufacturing and CDMO facilities
- AI-native biotech space requirements becoming mainstream
- Sustainability and ESG becoming table-stakes requirements
- Market differentiation between trophy and commodity assets
Long-Term Fundamentals:
- Demographic trends supporting pharmaceutical demand growth
- Technological innovation driving continued biotech investment
- Onshoring and supply chain resilience supporting manufacturing
- Life sciences cluster dynamics creating winner-take-most markets
- Specialized real estate expertise becoming increasingly valuable
For property professionals, the current market correction represents an opportunity to acquire assets at more reasonable valuations while positioning for the next growth cycle. However, success requires sophisticated understanding of property quality, tenant dynamics, and market positioning—precisely the expertise that comprehensive surveying and due diligence provides.
Conclusion: Mastering the Specialized Survey Approach
Surveying Niche to Essential Real Estate: Strategies for Labs, Cold Storage, and Life Sciences Sites in 2026 demands a fundamentally different approach than traditional commercial property assessment. The technical complexity, regulatory requirements, and rapidly evolving market dynamics require surveyors to develop deep specialized expertise across multiple disciplines—from mechanical engineering and environmental science to biotechnology trends and pharmaceutical manufacturing.
The transformation of life sciences real estate from niche to essential infrastructure is complete. With construction pipelines at historic lows, vacancy rates stabilizing, and manufacturing facilities demonstrating remarkable resilience, the sector offers compelling opportunities for those equipped to properly evaluate these specialized properties. The 220-basis-point expansion in cap rates since 2022 has reset valuations to more sustainable levels, creating entry points for strategic investors who understand how to assess quality and mitigate risk.
Key success factors for 2026 and beyond include:
- Technical Expertise: Developing comprehensive understanding of specialized building systems, regulatory requirements, and life sciences operations
- Market Intelligence: Tracking biotech funding trends, pharmaceutical onshoring, and emerging tenant requirements
- Risk Assessment: Implementing rigorous due diligence protocols that identify environmental, technical, and market risks
- Future Adaptability: Evaluating properties for conversion potential and alignment with evolving space utilization trends
- Value Optimization: Identifying opportunities to enhance property performance through sustainability, technology, and tenant alignment
Actionable Next Steps
For property professionals looking to capitalize on life sciences real estate opportunities:
🎯 Immediate Actions:
- Engage specialists for comprehensive commercial building surveys that address life sciences-specific requirements
- Review existing portfolio properties for conversion or repositioning opportunities
- Develop relationships with laboratory consultants, MEP engineers, and environmental specialists
- Study successful case studies in emerging markets like Philadelphia
📊 Strategic Planning:
- Evaluate geographic market opportunities beyond traditional coastal hubs
- Assess manufacturing vs. R&D property strategies based on risk tolerance
- Develop expertise in AI-native biotech space requirements and trends
- Create financial models that incorporate life sciences-specific metrics and risks
🔍 Knowledge Development:
- Pursue continuing education in life sciences facility design and operations
- Attend industry conferences focused on lab planning and pharmaceutical manufacturing
- Build networks with biotech real estate brokers, developers, and investors
- Stay current on regulatory changes affecting facility requirements
The life sciences real estate sector will continue to evolve rapidly as technology advances, tenant requirements shift, and market dynamics adjust. Property professionals who invest in developing specialized expertise, implement rigorous assessment protocols, and maintain market intelligence will be positioned to identify opportunities and mitigate risks in this essential but complex asset class.
Whether evaluating a potential acquisition, repositioning an existing asset, or providing advisory services to clients, the principles outlined in this guide provide a framework for success. The journey from niche to essential is complete—now comes the opportunity to master the specialized approaches that unlock value in this transformative sector.
For comprehensive property assessment services tailored to specialized real estate, consider engaging qualified professionals who understand the unique requirements of life sciences facilities. The investment in proper surveying and due diligence pays dividends through better acquisition decisions, optimized property performance, and reduced risk exposure in this dynamic market.
References
[1] Life Sciences – https://www.cbre.com/insights/books/us-real-estate-market-outlook-2026/life-sciences
[2] Jll 2026 Could Be A Realignment Year For Life Science Labs – https://www.rdworldonline.com/jll-2026-could-be-a-realignment-year-for-life-science-labs/
[3] Life Science – https://www.pwc.com/us/en/industries/financial-services/asset-wealth-management/real-estate/emerging-trends-in-real-estate-pwc-uli/property-type-outlook/life-science.html
[4] Us Life Sciences Long Term Outlook – https://www.clarionpartners.com/cpinsights/Documents/us-life-sciences-long-term-outlook.pdf
[5] Rethinking The Life Sciences Sector Beyond The Lab – https://www.perenews.com/rethinking-the-life-sciences-sector-beyond-the-lab/
[6] Life Sciences And Laboratory Equipment Market – https://www.skyquestt.com/report/life-sciences-and-laboratory-equipment-market